ResveratrolConsumer
avert online
consumer fraud
ResveratrolQuiz
test your knowledge
New E-Book
How the world got lost on
the road to an anti-aging pill
Subscribe to our newsletter to receive email notifications when new articles are posted.
June 30, 2011: by Bill Sardi
* SOD = superoxide dismutase
Genes they be — a Sirtuin family of seven “silent information regulators” that are guardians of the cell. Sirtuins have been linked with prolonged lifespan in various life forms, starting with yeast cells, fruit flies and roundworms.1
Sirtuin1 (also referred to as SIRT1) is a survival gene that is switched on when a living organism is deprived of food. Since the lifespan of calorie-restricted laboratory animals nearly doubles, researchers thought, for sure, they had struck biological pay dirt. Researchers thought any molecule that could turn on Sirtuin1 (trigger it to make proteins) could be an anti-aging molecule for humans. In fact, one Harvard genetics professor claimed Sirtuin1 was the “holy grail” of anti-aging2 and demonstrated in the laboratory that it could be activated by a red wine molecule called resveratrol.
All this was first announced in Nature magazine in late 20033, was extolled on the front pages of The New York Times4 and The Wall Street Journal5, and followed by a laboratory experiment which showed resveratrol prolonged the life of fat-fed mice6, and by 2010 a biotech company sold their experimental resveratrol-based drug to a major pharmaceutical company for $720 million.7
But that wasn’t quite how it all ended. A later study showed mega-dose resveratrol slightly shortened the lifespan of laboratory rats fed a standard-calorie diet, though it did dramatically improve other measurable health parameters largely by eradicating fatty liver.8
Then an MIT professor chimed in and said the pursuit of longevity via Sirtuin1 was a bit more complicated than first thought and that a calorie restricted diet didn’t uniformly up-regulate the Sirtuin1 gene in all organs and tissues.9 Dratted science never seems to cooperate just when humanity needed such a pill.
Then more confusion — the initial experiment which showed resveratrol activated Sirtuin1 was flawed. Turns out a fluorescent dye used in gene analysis foiled the experiment as it was found to be the agent that switched on Sirtuin1, not resveratrol.10 That didn’t negate the incomparable health benefits attributed to resveratrol, but it did negate Sirtuin1 as its primary gene target. Sounds like the pharmaceutical company ought to be asking for its money back. The major pharmaceutical company did announce it was not continuing further research and development of the SRT501 drug. Did the drug company remove SRT501 from its R&D pipeline to make sure it doesn’t compete with today’s disappointing prescription drugs.
But a month before the 2003 Nature magazine paper was published about Sirtuin1, researchers in Italy published an overlooked report concerning genetic data from a pool of humans over 100 years of age. These researchers found a relationship exists between longevity and genes located near the 11p15.5 chromosome. Five genes potentially involved in longevity were located and scrutinized. In this chromosomal region lies Sirtuin3, a gene that resides inside the energy power plants inside cells called mitochondria, whereas Sirtuin1 resides in the watery cytoplasm inside living cells.
A significant variation in survivorship was found among those individuals who exhibited a form of Sirtuin3. To date, among all seven Sirtuin genes, it is the only one genetically linked to lifespan in humans.11 But at the time, this discovery was overshadowed by the excitement for Sirtuin1.
Fast forward in time to 2011 – the science pointing to Sirtuin3 as a true anti-aging gene is mounting rapidly. As one research paper described it: “Sirtuin3 has recently stepped out of the shadow of Sirtuin1… mimicking calorie restriction.” Sirtuin3 was found to increase cellular respiration by 80% compared to 30% for Sirtuin1.12 Sirtuin1 gene’s own pied piper, Harvard researcher David Sinclair, filed for a patent in 2008 claiming activation of Sirtuin3 mimics the metabolic effect of endurance exercise training.13 No one noticed.
The whole tenor of anti-aging researchers has changed from the confusion and disappointment experienced with Sirtuin1. Usually reserved scientists are now saying things like “Sirtuin3 may be a central mechanism of aging retardation in mammals,14“ “Sirtuin3 may have profound implications for aging,15“ and that “a barrage of recent studies show that Sirtuin3 wards off the vicissitudes of aging.16“
To further corroborate the link between premature aging and lack of Sirtuin3 proteins, researchers have found that Sirtuin3 activity declines with advancing age.17
Surprisingly, if the Sirtuin3 gene is removed from laboratory mice, no overt signs of distress are observed. But if these same Sirtuin3-absent mice are subjected to biological or mental stress (food deprivation, excessive radiation, etc.), these stressed mice produce heightened levels of free radicals and decreased levels of cellular energy (ATP – adeno-triphosphate).18 Sirtuin3 is the antidote to harmful species of oxygen molecules known as free radicals, which are missing a key electron.
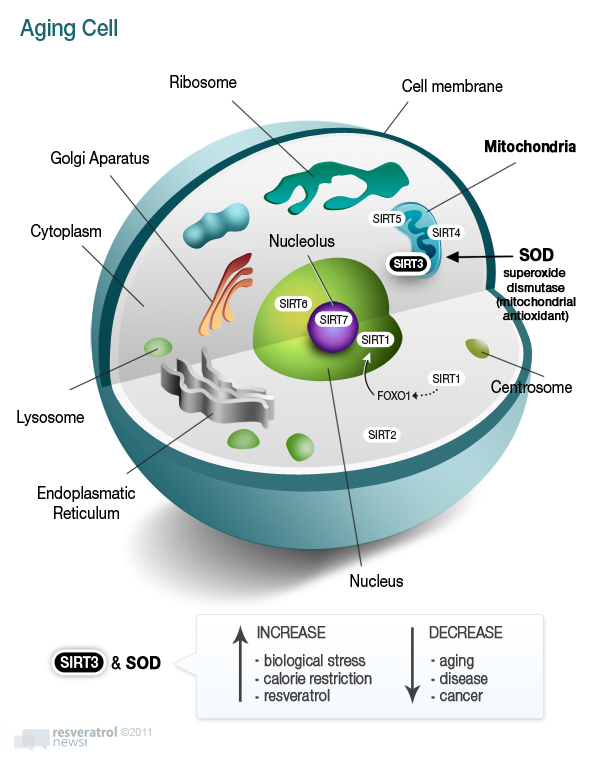
Why does Sirtuin3 appear to work against aging in a superior manner to Sirtuin1? The answer to this question may lie in the cellular compartment where it is found – the mitochondria. Sirtuin 3, 4 and 5 are found within the hundreds of mitochondrial compartments within living cells, which is where cellular respiration (conversion of oxygen to energy) takes place and where 90% of potentially destructive oxygen free radicals in the body are produced.
Sirtuin3 controls the internal (endogenous) antioxidant response to free radicals in the most critical of places – the mitochondria. It increases the activity of a natural endogenous antioxidant enzyme called superoxide dismutase (SOD) in the mitochondria.
The mitochondria are where 85-95% of oxygen used by cells is consumed to produce cellular energy and where over 90% of the destructive oxygen free radicals (oxidizing agents) are generated. By age 80 only about 4% of mitochondria are actually functioning at full capacity.19 This fact alone should indicate why Sirtuin3 may have such a profound and promising role as a single gene out of a library of about 25,000 genes. While according to one recent study human aging involves at least 295 genes20, Sirtuin3 appears to single-handedly counter the biological chaos that occurs within the mitochondria.
Think of a living cell with an outer fatty membrane holding in the watery cytoplasm. Within that cell is a nucleus that houses the genetic material – DNA. (See diagram below.) In the watery cytoplasm are hundreds of atomic power plants (mitochondria) that generate cell energy via respiration of oxygen. About 5% of that oxygen turns into potentially harmful oxygen free radicals, what is called oxidation or rusting. It’s like fires are burning in the hundreds of mitochondria in the cell with advancing age which must be extinguished by the manganese form of SOD via control by the Sirtuin3 gene. Put those mitochondrial fires out and cells in the brain, heart, liver, live longer and better withstand biological stress.
These facts do not negate the fact that the proper form of SOD (manganese-SOD) is also activated by resveratrol via the Sirtuin1 gene pathway.21 However, Sirtuin1 does not reside in the critical location that Sirtuin3 does.
Here are other known facts about this strategically placed gene:
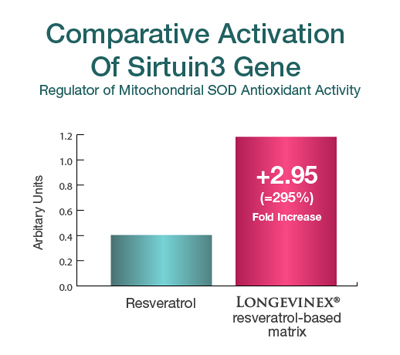
| Comparative Activation Of Sirtuin3 Gene Regulator of Mitochondrial SOD Antioxidant Activity |
||
| Arbitrary Units | Fold Increase | |
| Resveratrol | 0.40 | — |
| Longevinex® resveratrol-based matrix | 1.18 | +2.95 (295%) |
An intriguing animal study was conducted among mice that have been bred for hearing loss. If these animals are given a calorie restricted diet, despite their genetic predisposition to develop hearing loss, this sparse calorie diet completely prevented this occurrence. However, if the Sirtuin3 gene is removed from these animals and then they are given a limited calorie diet, the protection against hearing loss is completely negated.37
Corroboration of the beneficial effects of Longevinex® in humans have been received by researchers who have documented that older males experience hearing improvement as measured by audiological examination.38
Researchers are working feverishly on Sirtuin3 gene research. Will unfolding research result in a re-run of what occurred in 2003 with all the public fascination spawned by widespread news media coverage that surrounded resveratrol as a Sirtuin1 gene activator? Will the news media continue to ignore the stellar performance of the Longevinex® nutriceutical, which has many times now outperformed the $720 million SRT501 resveratrol drug?39,40,41,42 Will the National Institute on Aging launch a study to confirm whether resveratrol or a resveratrol-based matrix actually prolongs the life of laboratory animals? Stay tuned.
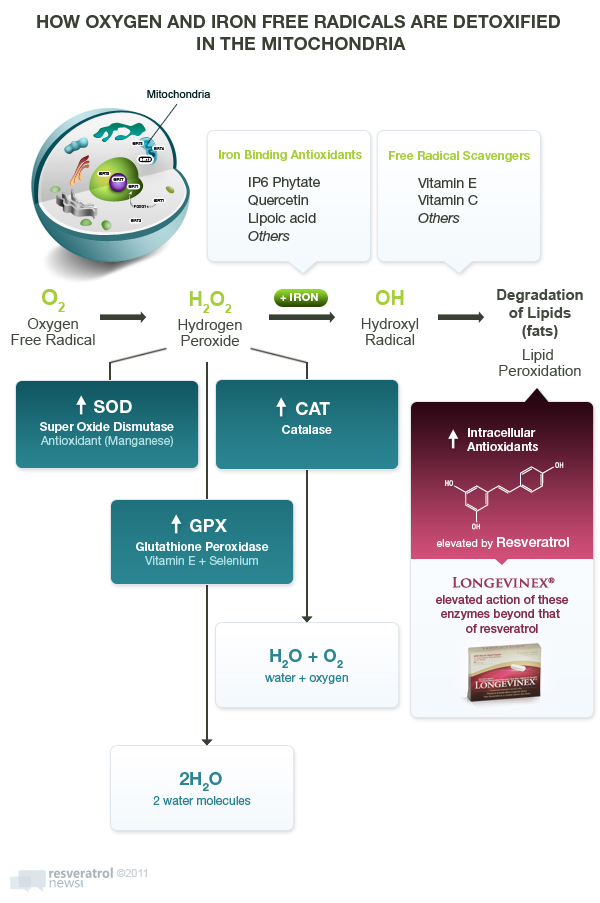
While manganese-SOD may be a primary protector of the mitochondria, it only converts troublesome destructive oxygen molecules called free radicals into hydrogen peroxide (H2O2), which itself is potentially destructive. Another internally-produced antioxidant enzyme, catalase, must convert hydrogen peroxide (H2O2) into harmless water (H2O). The process is O2 (reactive oxygen) -> H2O2 (hydrogen peroxide) + catalase -> = H2O (water).
Now if you read up on catalase, you would think it was the key antioxidant that produces longevity. In fact, in 2005 Science magazine published a report entitled “The Anti-Aging Sweepstakes: Catalase Runs for the ROSes” (ROS = reactive oxygen species).43
And there is strikingly convincing evidence for catalase as a key anti-aging antioxidant. For example, among mice bred to over-produce catalase, median lifespan was maximally increased by 5.0 to 5.5 months (these lab animals only live 2.0-2.5 years, so this is a 16-24% jump that in human terms would be equivalent to another 15-20 years of healthy living).44 Understand, that is five times greater predicted life extension than what can be achieved by elimination of cancer and heart disease in these animals. In mice bred to over-produce catalase, all of the age-related changes in heart function were significantly reduced.45
Catalase probably deserves as much or more attention if for nothing else than one molecule of catalase can break 40 million molecules of hydrogen peroxide every second, turning it to harmless water.
Also consider that while other antioxidants play a role in the mitochondria, they are often not able to deal with the large amount of oxidation (reactive oxygen species) and the mitochondria often lack sufficient catalase activity.46 With -38% chemically-induced reduction of catalase, normal cells display accelerated aging. The mitochondria become dysfunctional.47
When the human catalase gene is inserted in laboratory mice, they live longer, which is even more of an enticement to jump to the conclusion that catalase is THE longevity molecule.48
While consumers hear of the many protective properties of dietary and supplemental antioxidants such as vitamin C, vitamin E, coenzyme Q10 and others, three primary enzymatic antioxidants (catalase, superoxide dismutase and glutathione peroxidase) produced in living cells are overlooked.
Indeed, both selenium and vitamin E, the precursors for glutathione peroxidase, are required to protect mitochondria from damage by hydrogen peroxide.49
When researchers conducted a study to measure free radical production in the brains of different vertebrate animals, they found that SOD, catalase, glutathione peroxidase, the three major internally-produced antioxidants, along with vitamin C, correlated with maximum longevity. Low levels of free radical production and low antioxidant concentrations are a central finding in long-lived animals.50
However, there is contrary data that confounds a clear oxidant-antioxidant explanation of aging. High levels of oxidative damage in mitochondrial DNA do not decrease longevity in mice.51 Contrary to other experiments, when researchers intentionally induced overproduction of SOD, catalase, or combinations of the two, it didn’t result in extended lifespan of mice.52 In another mouse study, life-long reduction of MnSOD activity led to increased levels of oxidative damage to DNA and increased cancer incidence but did not appear to affect aging.53
Yet with all the praise for catalase, some researchers say the majority of studies using antioxidant strategies “do not support a clear role for mitochondrial oxidative stress or a vicious cycle of oxidative damage in the determination of lifespan in mice and furthermore do not support the free radical theory of aging.”54
In mice, the provision of increasing amounts of supplemental coenzyme Q10, a widely promoted antioxidant supplement, did not raise SOD, catalase or glutathione peroxidase antioxidant levels in the mitochondria and had no effect upon mortality.55
Of course, there is nothing better than human studies to test the antioxidant theory of aging. So recently researchers at the US Dept of Agriculture Human Nutrition Research Center on Aging at Tufts University enrolled 46 moderately overweight volunteers, age 20-42 years, and were given diets of differing reduced calorie load. Only glutathione peroxidase levels increased over a 6-month period of caloric restriction. No other antioxidants such as SOD or catalase were altered in blood plasma.56
Still, while scientific controversy reigns, researchers at the Division of Geriatrics, University of California at Los Angeles believe we are on the cusp of a breakthrough in the pursuit of human longevity and state that “the advancement of mitochondrially-targeted small-molecule antioxidants suggests the prospect of swift translation to human use.”57
Certainly, resveratrol rises to the top of a list of candidates as anti-aging molecules, with good reason. Examine these studies:In a lab dish, at very low concentration, resveratrol increases activity of the three major antioxidant enzymes, SOD, catalase and glutathione peroxidase, in cells obtained from the human retina.58 This occurs without provision of the nutrients (copper, zinc, manganese, vitamin E, selenium) necessary to produce these antioxidants.
It has been clearly demonstrated that resveratrol activates all three key antioxidant enzymes (glutathione, SOD and catalase) in arteries.59
Does resveratrol always shine in the laboratory? Well, in one experiment, at very low concentration, resveratrol had no effect upon key antioxidant enzymes such as catalase, glutathione peroxidase or copper-zinc variety of SOD in the lab dish. But, as researchers described it in their own words: “resveratrol dramatically and progressively induced mitochondrial manganese-SOD activity.” Two weeks into their experiment resveratrol increased manganese-SOD protein levels by 6-fold and Mn-SOD activity levels by 14-fold!60
Test tube studies however are not real live environments. So researchers put resveratrol to the test in mice, in what are called in vivo experiments. The lab mice were fed a standard calorie diet, a high-fat diet and were also fed via an osmotic pump to ensure the feeding was the same between animals. Unsurprisingly, resveratrol increased manganese-SOD protein-making by 140% and its activity by 75%.61
When one considers all of the factors that come into play in aging it would appear to be a daunting challenge to develop an anti-aging substance that would address all of the genes and biological processes involved, ranging from inflammation and oxidation to DNA breaks and enzyme control. But then again, there is resveratrol. Just examine how researchers explain the breadth of resveratrol’s biological influence:
Extensive research within the last decade has revealed that most chronic illnesses such as cancer, cardiovascular and pulmonary diseases, neurological diseases, diabetes, and autoimmune diseases exhibit dysregulation of multiple cell signaling pathways that have been linked to inflammation. Thus mono-targeted therapies developed for the last two decades for these diseases have proven to be unsafe, ineffective and expensive. Resveratrol, a polyphenol, has been shown to mediate its effects through modulation of many different pathways. This molecule has been shown to bind to numerous cell-signaling molecules such as multi drug resistance protein, topoisomerase II, aromatase, DNA polymerase, estrogen receptors, tubulin and F1-ATPase. Resveratrol has also been shown to activate various transcription factor (e.g; NFkappaB, STAT3, HIF-1alpha, beta-catenin and PPAR-gamma), suppress the expression of antiapoptotic gene products (e.g; Bcl-2, Bcl-X(L), XIAP and survivin), inhibit protein kinases (e.g; src, PI3K, JNK, and AKT), induce antioxidant enzymes (e,g; catalase, superoxide dismutase and hemoxygenase-1), suppress the expression of inflammatory biomarkers (e.g., TNF, COX-2, iNOS, and CRP), inhibit the expression of angiogenic and metastatic gene products (e.g., MMPs, VEGF, cathepsin D, and ICAM-1), and modulate cell cycle regulatory genes (e.g., p53, Rb, PTEN, cyclins and CDKs). Numerous animal studies have demonstrated that this polyphenol holds promise against numerous age-associated diseases including cancer, diabetes, Alzheimer, cardiovascular and pulmonary diseases. In view of these studies, resveratrol’s prospects for use in the clinics are rapidly accelerating. Efforts are also underway to improve its activity in vivo through structural modification and reformulation. Our review describes various targets of resveratrol and their therapeutic potential.”62
A question arises. If oxidation within the mitochondria increases with advancing age, and the mitochondria cease to function optimally in advanced age, what causes the progressive deterioration within the mitochondria? Genes do not run the show, they only respond to environmental cues within living cells.
This author maintains the accumulation of minerals, namely copper, iron and calcium, is what controls the rate of aging. Indeed, a recent report clearly describes how potentially destructive metals like copper, iron, even chromium and cobalt, produce metal-induced free radicals such as superoxide and the dreaded hydroxyl radical which then overwhelm antioxidant systems and damage DNA.63
Copper is the more powerful oxidant and it is of interest to note that while all metallic metals can induce potentially destructive free radicals, copper has been demonstrated to “overwhelm antioxidant defenses” (catalase, glutathione, SOD) more so than iron and other metallic minerals.64 As copper levels rise in the blood plasma, the risk for heart disease rises also.65 That cannot be said for iron. And resveratrol is “by far, the most potent chelator of copper.”66
It is interesting to note that females live longer than males in many species, including humans. And the mitochondria from females produce significantly less hydrogen peroxide than those from males and have higher levels of mitochondrial reduced glutathione, manganese superoxide dismutase, and glutathione peroxidase than males. Oxidative damage to mitochondrial DNA is also fourfold higher in males than in females. Researchers indicate “these differences may be explained by estrogens. Surgical removal of estrogen-producing ovaries abolishes the gender differences between males and females and estrogen replacement rescues the ovariectomy effect. The challenge for the future is to find molecules that have the beneficial effects of estradiol, but without its feminizing effects. Phytoestrogens or phytoestrogen-related molecules may be good candidates to meet this challenge.”67Resveratrol is a safe phytoestrogen.
What are we learning here? The messages to take home are large and many for longevinarians.
There is even another unheralded antioxidant enzyme, peroxiredoxin (Prx) that detoxifies hydrogen peroxide in the mitochondria and is linked to longevity. So we can’t pin all of our hopes on one gene or a single antioxidant.
Reply: Here is what the manganese-SOD molecule looks like:
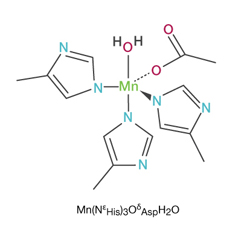
SOD is formed from three precursors – copper, zinc and manganese. Manganese-SOD constitutes approximately 10-15% of the total SOD activity in most tissues.
Manganese SOD appears to be a more powerful antioxidant than the copper or zinc-SODs under biologically stressed conditions.69
SOD activity is increased in response to biological stress. A frank deficiency of manganese and reduced manganese-SOD activity has been demonstrated to be deleterious to the heart. Provision of supplemental manganese has been shown to be therapeutic.70 However, the heart-protective properties of manganese were only demonstrated in manganese-deficient animals compared to manganese-sufficient animals.71
Higher intake of manganese from the diet, but not necessarily supplements, is associated with lower risk for brain disease.72 Inorganically-bound manganese in drinking water is potentially more troublesome than bound forms of manganese in the diet.73 In animals, chronic exposure to manganese and other heavy metals in drinking water was counterproductive and actually decreased SOD activity over the long term.74
Supplemental manganese has drawn cause for concern in multivitamins.75 The estimated safe daily intake for manganese is 2-5 mg per day, however dosage range for side effects begins at just 4.2 mg/day for a 70-kilogram/160-lb individual. So the potentially problematic dose falls within the so-called safe range.76
A high intake of manganese when accompanied with iron is also of concern in relation to age-related brain disease.77 With advancing age, metallic metals accumulate in living tissues. The accumulation of manganese may be deleterious.78
The Western diet appears to generally provide sufficient amounts of manganese.79
Provision of oral SOD itself without biological stress may sometimes be problematic. Manganese is a heavy metal that is only needed in minute amounts to produce SOD. Excessive activation of manganese-SOD, while anticipated to be increased under biological stress, may not be beneficial when constantly increased when biological stress is low or absent.80
Reply: SOD is a antioxidant enzyme that is rapidly degraded by gastric acids when ingested orally. Even enteric coated tablets do not adequately protect SOD and SOD supplements are said to produce no pharmacologic activity when taken orally. Oral SOD was found to ineffectively raise SOD levels in laboratory mice.81 However, this is a dated study and only used low (less than 1mg) doses.
Oral SOD may be of some value when administered directly into tissues. The provision of oral SOD directly into the gut reduced oxidative stress in animals with colitis. A 910-mg equivalent human dose (13 mg/kilogram for a 70 kilogram/160-lb human) was employed.82
Various brands of oral SOD are sold in retail shops. The bioavailability of oral SOD is not equivalent to what is employed in the research laboratory where injectable SOD is often used. Oral SOD is poorly bioavailable unless special encapsulation methods are employed.83 Manganese-SOD has a high molecular weight of around 81,000 Daltons and would be expected to be poorly absorbed.84 For comparison, the molecular weight of readily absorbed vitamin C is 176 Daltons. Low molecular weight SOD has been successfully employed in animal studies, but was administered intravenously.85 The trick is to activate SOD in the mitochondria, not to assume that oral SOD makes its way to remote tissues beyond the digestive tract.
References
To access abstract, click on PubMed (National Library of Medicine) master identification number (PMID) at end of citation.
Copyright 2011- Bill Sardi
Posted in Resveratrol
Add comments »