ResveratrolConsumer
avert online
consumer fraud
ResveratrolQuiz
test your knowledge
New E-Book
How the world got lost on
the road to an anti-aging pill
Subscribe to our newsletter to receive email notifications when new articles are posted.
December 13, 2018: by Bill Sardi
CLINICIAN’S GUIDE TO RESVERATROL DISPELS INACCURACIES AND MYTHS ABOUT RESVERATROL
FOR HEALTH PROFESSIONALS
This report pertains to:
COMMENTARY ON:
Re: Health Effects of Resveratrol: Results from Human Intervention Trials
Corresponding author: Rosa M. Lamuela-Raventos at lamuenta@ub.edu
Published: NUTRIENTS Dec. 3, 2018 doi: 10:3390/nu10121892
Dear doctor: Why aren’t you taking resveratrol? I hope it isn’t for any of the bogus scientific objections modern medicine postulates, such as poor bioavailability, confusion over dosage or that there is insufficient evidence and is therefore not proven yet (but not disproven).
The purpose of this responsive report is to provide critical comment of a recently published overview of resveratrol, a natural molecule that has drawn considerable scientific interest over the past decade. The scope of the overview published in the journal NUTRIENTS[1] was to assess the limited number of published human studies. However, upon reading the report I discovered it did not provide an accurate or complete enough picture of the entire body of published studies involving resveratrol. In particular the all- important issue of dosage was not adequately addressed.
The most widespread scientific misconception of resveratrol is that it has “poor bioavailability.”[2],[3] There also was confusion in terminology between absorption and bioavailability, the latter being the ability of resveratrol in the blood circulation to pass through the liver in a free unbound state without conjugation with sulfate or glucuronate vs. absorption which takes place in the gut.
According to Walle[4], about 70% of trans resveratrol is absorbed. Almost all resveratrol is eventually metabolized as it passes through the liver, though accompanying quercetin[5] or piperine[6] allow a few more passages through the liver before almost all is bound to sulfate and glucuronate. It would be more accurate to say piperine/quercetin facilitates more immediate bioavailability of resveratrol.
Researchers at the University of Leicester say further investigation shows resveratrol can be taken up into cells after it has been metabolized in the liver to res-sulfate. In fact, resveratrol may be more effective once in its liver metabolized form.[7] Res-sulfate was demonstrated to slow the rate of cancer by causing them to digest their own internal parts and halting their replication. The lead researcher says this helps to explain why resveratrol works at such low-dose concentrations. It may not be necessary to deliver biologically active doses to people, said researchers.[8]
The claim made that pterostilbene, a methylated form of resveratrol, is more bioavailable is a bit misleading. Yes, true, but it being a free unbound (un-liver metabolized) molecule means it will only have a short half-life of ~14 minutes according to Walle. The advantage of a 9-hour half-life when conjugated with glucuronate or sulfate is traded for immediate bioavailability.
Given that resveratrol metabolites are biologically active, the claim that resveratrol is not biologically available is moot. Methylated resveratrol is better absorbed intestinally but not longer-acting.[9]
Furthermore, the claim that liver metabolism renders non-bioavailable resveratrol is countered by the fact some drugs are intentionally glucuronidated as a delivery system.[10] Glucuronidase is up to 16 times more active at sites of inflammation, infection and malignancy and releases glucuronidated drugs and molecules like resveratrol from glucuronate, thus delivering drugs and free unbound resveratrol at the right time and place.[11] The same holds true for resveratrol when it has been conjugated to sulfate in the liver. Sulfatase enzyme activity unlocks resveratrol from sulfate, making it bioavailable.[12]
A great deal of the literature about resveratrol repeats the mistaken idea that resveratrol is not bioavailable. This is despite published studies in both animals and humans that confirm there are systemic effects even in the brain and eyes which requires passage through the blood/brain-ocular barriers. For example, resveratrol has been found in tissue samples of the retina of humans.[13] Resveratrol inhibits destructive new blood vessel formation (angiogenesis/neovascularization) in human cases of wet macular degeneration sufficient to restore visual function to very aged patients.[14]
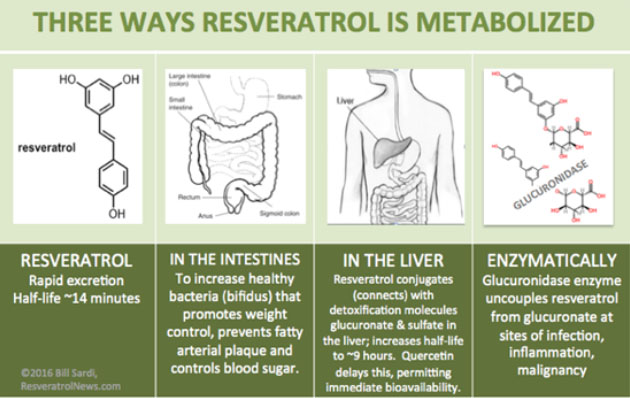
There are numerous published reports how to make resveratrol more bioavailable by rearranging the molecule or complexing it with other molecules which appear to be unnecessary given the above information.
Furthermore, the idea of the need for nanosized resveratrol is a bit questionable. Cells with a mass of less than 100 nanometers [15](billionth of a meter) in one direction can absorb molecules and pass through the blood brain barrier.[16] Many natural molecules already are smaller than 100 nanometers and don’t require nanosizing to enter cells. Many of these small molecules are available today as herbal extracts and, so far, have outperformed synthetic analog (look-alike) molecules.
|
Natural Molecule |
Avg. size in nanometers |
Natural Source |
|
EGCG |
260 |
Green tea |
|
Curcumin |
45-80 |
Turmeric spice |
|
Ellagic Acid |
30 |
Pomegranate |
|
Ferulic Acid |
100-200 |
Rice bran |
|
Quercetin |
50-270 |
Onions, apple peel |
|
Resveratrol |
78-180 |
Grapes, wine |
| Source: Nair, H.B.; Sung, B.; Yadav, V.R., Kannappan, R.; Chaturvedi, M.M., Aggarwal, B.B. Biochemical Pharmacology 2010, 80, 1833-1843. | ||
In regard to the comment that “some participants reported one or more adverse events, such as gastrointestinal symptoms including nausea, flatulence, bowel motions, abdominal discomfort, loose stools and diarrhea,” no explanation is given as to the obvious reason why these side effects sometimes occur.
Weak botanical extracts of resveratrol (10%, 25%, 50%) contain unwanted emodin which induces loose stool and a laxative effect (confused for diarrhea, which is expulsive elimination).[17] For example, in one small pilot study 6 of 8 subjects were reported to have experienced “diarrhea.”[18] Purer extracts (~80%+) or fermented (synthetically produced) sources (98%+) should reduce or eliminate the emodin problem.
As with all dietary supplements, they should be taken with food, not on an empty stomach, as sufficient digestive juices may not be produced without food for proper digestion and absorption.
The American Association of Poison Control Centers data year on end list no reports of hospitalizations or deaths associated even with the misuse of resveratrol.[19]
The statement that resveratrol is safe at almost any dose (up to 5000 milligrams) is accurate in regard to no hospitalizations or deaths. But that does not mean it is without side effects nor give indiscriminate license to use mega-doses.
At the writing of this commentary there are 531 brands of resveratrol-based dietary supplements listed by Natural Medicines Comprehensive Database (NMCD).[20] The NMCD report says resveratrol is “likely safe” with doses up to 3000 mg/day being well tolerated though side effects include mild gastrointestinal discomfort, loose stools, headache likely from anemia- author’s comment) with no toxicity up to 4000 mg/day. But these are unnecessarily high doses that may be counterproductive.
The NMCD says there are theoretical interactions with drugs due to cytochrome p450 involvement that may not be compatible with anticoagulant drugs (blood thinners). Though this author, having over a dozen years of experience marketing a resveratrol dietary supplement, has not received a single confirmed report of nose bleed or hemorrhage with concomitant use of blood thinners and resveratrol. By personal experience, clotting time is noticeably prolonged by a minute or two with use of a compression bandage over a bleeding wound.
According to data presented at the NMCD website, peak plasma oral doses are reached in 0.5 to 1.5 hours. A single 5-gram dose results in peak plasma levels ~2.4 micromole/liter with 5-micromole said to be needed for chemo-preventive effects. But such a high blood concentration is needed for a pro-oxidant cell-killing cytotoxic effect for the treatment of cancer, not for prevention or daily use to promote health.
Doses ranging from 25-500 mg produce plasma levels of 5-14 nanograms/milliliter with liver metabolites almost 10 times those levels. Micronization increases plasma concentration (by 3.6-fold in one study).[21]
Excretion with low doses (0.3 mg/kilogram body weight) is over 50% in urine within 24 hours and with higher doses of 1 mg/kilogram, ~25% is excreted within 24 hours. In humans, 77% of all urinary resveratrol metabolites are excreted within 4 hours after ingestion of 5 grams of resveratrol. This serves as evidence the body perceives resveratrol as a potential toxin (molecular mimic of starvation) which is why it is extensively detoxed and rapidly shuttled to the kidneys for elimination.
Indeed, 5000 mg did induce kidney failure and other serious side effects among patients with multiple myeloma who are vulnerable to kidney problems apart from resveratrol.[22]
While resveratrol is safe over a wide dosage range, it is not totally without side effect. Unpublished side reactions logged by a resveratrol pill company I manage include Achilles heel tendonitis; frontal (behind the eye) headache, usually associated with menstruating females with anemia; and skin rash, anxiety and flu-like symptoms similar to those reported with TNF-inhibiting drugs (resveratrol being a strong TNF inhibitor). While resveratrol may produce side effects as observed in subjects undergoing TNF inhibition (e.g. Etanercept/ Enbrel), a confounding report indicates resveratrol is a strong inhibitor of C-reactive protein but not TNF in humans.[23]
Toxicologist Ken Eagle writes of the ability of polyphenols like resveratrol to inhibit sulfotransferase enzymes that keep a lid on stress hormones produced from the adrenal glands. Modest dose polyphenols would mildly increase mental alertness but excessive dose might cause the heart to race or flutter (fibrillation), migraines to occur, blood pressure to rise and anxiety to be experienced.[24],[25] Reports of such side effects have been reported to this author. Though resveratrol is also considered a remedy for atrial fibrillation in models of heart failure and chronic inflammation.[26],[27] Avoidance of mega-dosing may be important for this application.
Resveratrol also inhibits detoxification via cytochrome p450 enzymes and thus, when consumed with medications, can heighten the effect of drugs to the point of side effects (for example, drop blood pressure too far if taking anti-hypertensive medications), but these reactions are transient and self-limiting as all resveratrol will rapidly be conjugated with glucuronate and sulfate.
In lab dishes and the animal lab researchers tend to employ ultra-mega doses of resveratrol to prove effect. These are often impractical doses for humans.[28]
In regards to cancer prevention (not therapeutic treatment for a cure) only among mice given low doses of resveratrol typical of those found among wine drinkers, compared to doses 200-times higher, were intestinal adenomas suppressed more than the higher dose.[29] The more-is-better approach may not fit use of resveratrol for cancer prevention.
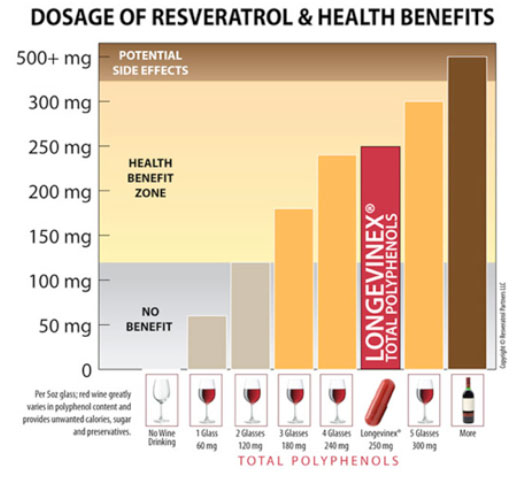
Recognize wine by virtue of fermentation concentrates polyphenol molecules 1000-fold from micrograms to milligrams. There is no magic in grape juice as there is in wine. The average red wine can be expected to provide ~1.9 ±1.7 mg resveratrol per 1-liter bottle.[30] One liter is = to five 5-oz. glasses. So that is very little resveratrol.
Epidemiologic studies show the consumption of 3-to-5 5-oz glasses of wine per day dramatically reduces mortality from coronary artery disease (classical U-shaped risk curve) which could not possibly be explained by resveratrol alone.
It was professor Roger Corder of the William Harvey Research Institute in London who has said the healthy properties of wine must come from all of the polyphenols in red wine including anthocyanidins[31] which average in aged wine bottles at ~60 mg per 5-oz. glass.
Intake of 3-to-5 glasses of wine provides an estimated ~180-300 milligrams of total polyphenols. Of course, this borders on chronic inebriation given the alcohol content of wine. Consumption of resveratrol + other polyphenols without the alcohol is desirable and can be achieved with dietary supplements.
As confirmation, men given dealcoholized wine experienced a decline in blood pressure (6/2 points systolic/diastolic pressure) equal to that of antihypertensive drugs while the men who drank regular wine experienced only a slight drop in blood pressure.[32]
In the 1990s North America had a coronary artery disease mortality rate of 240 per 100,000 versus just 91 per 100,000 for the wine-drinking French. In the 1990s epidemiologists reported the consumption of 3-to-5 glasses of red wine produced a U-shaped risk curve.[33] Teetotalers and over-imbibers did not derive a mortality reduction.
The above information provides important clues as to proper dosage of resveratrol or resveratrol combined with other polyphenols.
A corroborative dosing study in animals is telling. Blood circulation to animal hearts was experimentally halted then resumed resulting in reperfusion injury to heart muscle. Animals were pre-dosed with 2.5, 5.0, 25.0 and 50.0 mg resveratrol per kilogram of body weight equivalent in human terms in a 70-kilogram (154-lb.) human to 175, 350, 1750 and 3500 mg.
Resveratrol between 2.5-5.0 mg/kilogram (175-350 mg in 70-kilogram/ 154-lb. humans) prevented damage to heart muscle[34] whereas 25.0-50 mg/kilogram (1750-3500 mg in humans) increased the size of the heart attack and cardiac fibrosis (scarring)! This study is instructive as to antioxidant vs. pro-oxidant dose. Resveratrol did not induce a heart attack, but when one was experimentally induced, a pro-oxidant dose increased tissue damage and fibrosis (scarring) of heart muscle.
Pay particular attention to the fact the 175-350 mg dose of resveratrol employed experimentally in the animal lab matched the 180-300 mg dosage range in 3-to-5 glasses of wine observed to produce a decline in coronary artery disease mortality among wine drinkers.
An exception was noted in a multi-ingredient resveratrol-based dietary supplement (Longevinexâ) where 2800 mg human equivalent dose (a dose that normally “kills” the rodent heart) did not exert any toxicity due to a trade secret the company utilizes to negate mega-dose toxicity. This created the first known L-shaped risk curve and posits this product as preferentially the safest among resveratrol pills. Longevinexâ remained cardioprotective even at 100 mg/100-gram body weight – a dose that killed 100% of the animal hearts when tested with pure resveratrol.[35]
A case for more than dietary intake level but relatively low-dose resveratrol can be made. Mice fed 4.9 mg/kilogram of body weight (343 mg human equivalent) resveratrol, considered a modest supplemental dose, molecularly mimicked the effects of a lifespan/health span-doubling calorie restricted diet.[36]
A 12-week study of laboratory mice utilizing relatively low-dose resveratrol ~1.5 mg/kilogram of body weight (~100 mg human equivalent dose) combined with quercetin and IP6 rice bran phytate (Longevinexâ) activated 1711 genes in a global gene array analysis compared to resveratrol 225 genes and calorie restriction 198 genes. Life-long calorie restriction significantly differentiates 831 genes and Longevinexâ activated 677 of those 831 genes (81.4%), the closest mimic of CR to date.[37] This appears to be an example of molecule synergism.
Resveratrol in nature and in particular in a concentrated (fermented) form in wine is never encountered by itself but in a combination of other polyphenols and other antioxidants. Molecular synergism accomplished with a low-dose combination of polyphenols in a dietary supplement would appear to be advantageous.
There are other examples of molecular synergism.
If you prescribe resveratrol pills for your patients you may be prescribing a dud.
Resveratrol needs to be protected from light and heat.[40] Otherwise it is degraded into a less active form (cis resveratrol). Microencapsulation enfolds resveratrol in plant starches and dextrins to protect from light. Otherwise you may be prescribing a dud pill for your patients. I had various brands of resveratrol pills tested and they contained a significant amount of degraded (cis) resveratrol rather than the active (trans) resveratrol. My company was sued by two competitors for revealing that (ConsumerLab eventually tested those two products and found they hardly had any resveratrol in them). Preservation in foil packaging is also recommended. Also, the inclusion of beta cyclodextrin solubilizes resveratrol and stabilizes the molecule and is desirable in dietary supplements.[41]
Too often resveratrol used in dietary supplements is of low quality and purity, having enough emodin to produce gastrointestinal side effects and loose stool (take with food to avoid these side reactions).
The most acute time for doctors to prescribe resveratrol is after their own heart attack. Historically resveratrol was introduced as a remedy for heart disease. Given that wine is the most concentrated food source of resveratrol it was Dr. Serge Renaud of France who appeared on the Sixty Minutes television show to say far fewer wine-drinking Frenchmen were dying of coronary artery disease than North Americans at the time even though these Frenchmen had higher cholesterol levels (the so-called French Paradox).[42]
Should resveratrol be employed post-heart attack in lieu of or in addition to the regular salvo of drugs (statins, diuretics, beta blockers, ACE inhibitors) standardly prescribed? Even among post-heart attack patients prescribed beta blockers, statin drugs and aspirin, mortality rates remain high (24% a 1 year; 51% at 5 years; 65% at 8 years.[43]
You aren’t going to prescribe yourself a baby aspirin like you do your own patients are you? About half of the people experiencing a heart attack were taking an aspirin tablet on the day of their event. An authoritative report published in The American Journal of Medicine indicates a baby aspirin (81 mg) is too weak to prevent a heart attack.[44] A standard 325 mg aspirin tablet is adequate but is fraught with the risk for gastric bleeding and brain hemorrhage. The US Food & Drug Administration has twice denied applications to approve aspirin for the primary prevention of cardiovascular event in any population.[45] If you are still prescribing a baby aspirin to prevent heart attacks you are about 20 years behind the times since the US Preventive Services Task Force first issued that recommendation.
You aren’t going to prescribe a statin drug for yourself are you? Only 1 in 250 statin drugs users will avert a non-mortal heart attack over a 5-year period. Statin drugs don’t reduce the risk for mortal heart attacks.
The best form of protection against a second heart attack is to activate cardioprotection, that is, to induce endogenous enzymatic antioxidant (glutathione, SOD, catalase) to pre-protect the heart should a blockage in a coronary artery occur.[46]
Oh, you ask, is there a double-blind placebo-controlled study to prove that? No, it would be unethical to give resveratrol pills to a group of patients and an inactive placebo pill to another group and count how many die in each group. So, we have to rely upon animal studies.
In the animal lab researchers showed resveratrol had a profound effect upon post infarction (heart attack) health, improving left ventricle pumping power, decreasing fibrosis (scarring), reducing hypertrophy (enlarged heart).[47] Remarkably resveratrol has even been shown to reduce injury to heart muscle following a heart attack.[48]
Will any brand of resveratrol pill do here? Only one brand of resveratrol has undergone testing in the animal lab to confirm it does in fact limit damage to heart muscle in the event of a heart attack.[49],[50]
Ah, as a physician you await convincing human studies before you venture to take such an unproven remedy. Researchers in Europe employed just 10 milligrams of resveratrol daily among 40 post-infarction patients and report the pumping power of the left ventricle of these patients improved, the ability of their arteries to widen to control blood pressure (flow-mediated dilatation) improved, fewer red blood cells clumped together to potentially form clots in coronary arteries and red blood cells were less deformed.[51]
Would you as a physician drink wine after your first heart attack?
Doctors in Italy put that idea to the test and found moderate wine intake improved cardiac function, ejection fraction (pumping power), blood flow and reduced markers of inflammation.[52]
I know physicians often stare down their heart attack patients and warn them they will have another heart attack and die if they don’t take their statin cholesterol-lowering drug. But a recent study shows even the most aggressive cholesterol reduction with statin drugs following a first heart attack would result in negligible reductions of risk for a future heart attack or cardiac death.[53]
Compare that study with another recent study which showed those post-heart-attack patients who took a high-dose multivitamin and mineral complex were 54% less likely to experience a mortal heart attack, stroke, or recurrent heart attack than non-vitamin users.[54]
It must be particularly gnawing to know you went to medical school and attended all those required continuing education courses only to find out you’ve been practicing outdated medicine. But certainly, avail yourself of resveratrol even if it isn’t included in current treatment guidelines.
You may not prescribe resveratrol for your patients but you don’t want to wait till you have your first heart attack to start taking resveratrol, do you?
Bill Sardi, Resveratrol Partners LLC, dba LONGEVINEX
www.longevinex.com
[1] Ramirez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Munoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M. Health effects of resveratrol: results form human intervention trials. Nutrients, 2018, 10, E1892.
[2] Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, E91.
[3] Espinoza, J.L.; Kurokawa, Y.; Takami, A. Rationale for assessing the therapeutic potential of resveratrol in hematological malignancies. Blood Rev. 2018, S0268-960X, 30124-30128.
[4] Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E. Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos 2004, 12, 1377-1382.
[5] De Santi, C.; Pietrabissa, A.; Mosca, F.; Pacifica, G.M. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica 2000, 30, 1047-1054.
[6] Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169-1176.
[7] Resveratrol remains effective against cancer after the body converts it. Oct. 2, 2013, Medicalxpress.com accessed Dec. 13, 2018.
[8] Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R., Steward, W.P.; Gescher, A.J.; Brown, K. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Science Translational Med. 2013, 5, 205ra133.
[9] Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 10, 1786-1792.
[10] Kaivosaari, S. N-glucuronidation of drugs and other xenobiotics. Univ. Helsinki, dissertation, 2010.
[11] Wang, L.X.; Heredia, A.; Song, H.; Zhang, Z.; Yu, B.; Davis, C.; Redfield, R. Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity. J.Pharm. Sci. 2004, 93, 2448-57.
[12] Miksits, M.; Wicek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jager, W. Antitumor activity of resveratrol and its sulfate metabolites against human breast cancer cells. Planta Med. 2009, 75, 1227-1230.
[13] Wang, S.; Wang, Z.; Yang, S.; Yin, T.; Zhang, Y.; Qin, Y.; Weinreb, R.N.; Sun, X. Tissue distribution of trans-resveratrol and its metabolites after oral administration in human eyes. J. Ophthalmology 2017, 2017, 4052094.
[14] Richer, S.; Patel, S.; Sockanathan, S.; Ulanski, L.J. Jr.; Miller, L.; Podella, C. Resveratrol based oral nutriti9onal supplement produces long-term beneficial effects on structure and visual function in human patients. Nutrients 2014, 6, 4404-4420.
[15] Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of anti-inflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833-43.
[16] Wilson, B. Therapeutic compliance of nanomedicine in Alzheimer’s disease. Nanomedicine 2011, 6, 1137-1139.
[17] Zheng, Y.F.; Liu, C.F.; Lai, W.F.; Ziang, Q.; Li, Z.F.; Wang, H.; Lin, H.; The laxative effect of emodin is attributable to increased aquaporin 3 expression in the colon of mice and HT-29 cells. Fitoterapia 2014, 96, 25-32.
[18] La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449-54.
[19] National Poison Data System Annual Reports, years 2012-2016, American Association of Poison Control Centers, online athttps://aapcc.org/annual-reports
[20] Natural Medicines Comprehensive Database, accessed Dec. 11, 2018.
[21] Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases- safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419-1425.
[22] Popat, R.; Flesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT510 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. British Journal Haematology 2012, 160, 714-717.
[23] Haghighatdoost, F.; Hariri, M.; Can resveratrol supplement change inflammatory mediators? A systematic review and meta-analysis on randomized clinical trials. Eur. J. Clin. Nutr. 2018, July 16.
[24] Eagle, K, Toxicological effects of red wine, orange juice, and other dietary SULT1A inhibitors via excess catecholamines. Food Chem Toxicol 2012, 50, 2243-49.
[25] Eagle, K. Hypothesis: holiday sudden cardiac death: food and alcohol inhibition of SULT1a enzymes as a precipitant. J. Appl. Toxicol. 2012, 32, 751-55.
[26] Chong, E.; Chang, S.L.; Hsiao, Y.W.; Singhal, R.; Liu, S.H.; Leha, T.; Lin, W.Y.; Hsu, C.P.; Chen, Y.C.; Chen, Y.J.; Wu, T.J.; Higa, S.; Chen, S.A. Resveratrol, a red wine antioxidant, reduces atrial fibrillation susceptibility in the failing heart by P13K/AKT/eNOS signaling pathway activation. Heart Rhythm 2015, 12, 1046-1056.
[27] Sutanto, H, Dobrev, D, Heijman, J. Resveratrol: an effective pharmacological agent to prevent inflammation-induced atrial fibrillation. Naunyn Schmiedebergs Arch. Pharmacol. 2018; 39, 1163-1167.
[28] Scott, E.; Steward, W.P.; Gescher, A.J.; Brown, K. Resveratrol in human cancer chemoprevention – choosing the ‘right’ dose. Mol. Nutr. Food Res. 2012, 56, 7-13.
[29] Scott, E.; Cai, H.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; James, M.; Howells, L.; Ognibene, T.; Malfatti, M.; Goldring, C.; Kitteringham, N.; Steward, W.P.; Gescher, A.J.; Brown, K. Less is more for cancer chemoprevention; evidence of a non-linear dose response for the protective effects of resveratrol in humans and mice. Sci. Trans. Med. 2015, 7, 298ra117.
[30] Weiskirchen, S.; Weiskirchen, R. Resveratrol: how much wine do you have to drink to stay healthy? Adv. Nutr 2016, 7, 706-18.
[31] Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.;Wood, E.G., Carrier, M.J., Crozier, A. Oenology: red wine procyanidins and vascular health. Nature 2006, 444, 566.
[32] Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martinez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; Estruch, R. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide. Circulation Research 2012, 111, 1065-1068.
[33] Gronbaek, M.; Deis, A.; Sorensen, T.I.; Becker, U.; Schnohr, P.; Jensen, G. Mortality associated with moderate intakes of wine, beer or spirits. Br. Med. J. 1995, 301, 1165-69.
[34] Dudley, J.; Das, S.; Mukherjee, S.; Das, D.K. Resveratrol, a unique phytoalexin in red wine, deliver either survival signal or death signal to the ischemic myocardium depending upon dose. J. Nutr Biochem. 2009; 20, 443-52.
[35] Juhasz, B; Mukherjee, S.; Das, D.K. Hormetic response of resveratrol against cardioprotection. Exp. Clin. Cardiol. 2010, 15, 134-38.
[36] Barger, J.L.; Kayo, T.; Vann, J.M.; Arias, E.B.; Wang, J.; Hacker, T.A.; Wang, Y.; Raederstorff, D.; Morrow, J.D.; Leeuwenburth, C.; Allison, D.B..; Saupe, K.W.; Weindruch, R.; Prolla, T..A. A low dose of dietary resveratrol partically mimics calorie restriction and retards aging parameters in mice. PLos One 2008, 3, 2264.
[37] Barger, J.L.; Kayo, T.; Pugh, T.D.; Prolla, T.A.; Weindruch, R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp. Gerontol. 2008, 43, 859-66.
[38] Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Caubano, L.A.; Dharmawardhane, S.F.; Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl. Oncol. 2008, 1, 19-27.
[39] Mertens-Talcott, S.U., Percival, S.S.; Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Letters 2005, 218, 141-151.
[40] Chen, Y.B.; Sun, B.X.; Chen, J.X Study on the stability of resveratrol in rhizome polygoni cuspidate.. Journal of Chinese Medicinal Materials 2007, 30, 805-807.
[41] Silva, F.; Figueiras, A.; Gallardo, E.; Nerin, C.; Domingues, F.C. Strategies to improve the solubility and stability of stilbene antioxidants: a comparative study between cyclodextrins and bile acids. Food Chem. 2014, 145, 115-125.
[42] Renaud, S.; Gueguen, R. The French Paradox and wine drinking. Novartis Found. Symp. 1998, 216, 208-217.
[43] Kochar, A.; Chen, A.Y.; Sharma, P.P; Pagidipati, N.J.; Fonarow, G.C.; Cowper, P.A.; Roe, M.T.; Peterson, E.D.; Wang, T.Y. Long-term mortality of older patients with acute myocardial infarction treated in US clinical practice. J. Am. Heart Assoc. 2018, 7, e007230.
[44] Dalen, J.E. Aspirin for the primary prevention of stroke and myocardial infarction: ineffective or wrong dose? The Am. J. Med. 2010, 123, 101-102.
[45] Hissett, J.; Folks, B.; Coombs, L.; LeBlanc, W.; Pace, W.D. Effects of changing guidelines on prescribing aspirin for primary prevention of cardiovascular events. J. Am. Board Family Med. 2014, 25, 78-86.
[46] Dernek, S.; Ikizler, M.; Erkasap, N.; Ergun, B.; Koken, T.; Yilmaz, K.; Sevin, B.; Kaygisiz, Z.; Kural, T. Cardioprotection with resveratrol pretreatment: improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand. Cardiovasc. 2004, 38, 245-254.
[47] Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxidative Med. Cellular Longevity 2017, 2017, 6819281.
[48] Goh, S.S.; Woodman, O.L.; Pepe, S.; Cao, A.H.; Qin, C.; Ritchie, R.H. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxidant Redox Siognal. 2007, 9, 101-113.
[49] Mukherjee S., Ray, D.; Lekli, I, Bak, I.; Tasaki, A.; Das, D.K. Effects of Longevinex (modified resveratrol) on cardioprotection and its mechanisms of action. Can. J. Physiol. Pharmacol. 2010, 88, 1017-25.
[50] Mukhopadhyay, P.; Mukherjee, S.; Ahsan, K.; Bagchi, A.; Pacher, P.; Das, D.K. Restoration of altered micro-RNA expression in the ischemic heart with resveratrol. PLoS One 2010, 5, e15705.
[51] Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin. Hemorrheol. Microcirc. 2012, 50, 179-187.
[52] Marfella, R.; Cacciapuoti, F.; Siniscalchi, M.; Sasso, F.C.; Marchese, F.; Cinone, F.; Musacchio, E. Marfella, M.A.; Ruggiero, L.; Chiorazzo, G.; Liberti, D.; Chiorazzo, G., Nicoletti, G.F.; Sardu, C.; D’Andrea, F.; Ammendola, C.; Verza, M.; Coppola, L. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabetic Medicine 2006, 23, 974-981.
[53] Alter, D.A.; Tu, J.V.; Koh, M.; Jackevicius, C.A.; Austin, P.C.; Rezai, M.R.; Bhatia, R.S.; Johnston, S.; Udell, J.A.; Ko, D.T. Projected real-world effectiveness of using aggressive low-density lipoprotein cholesterol targets among elderly statin users following acute coronary syndromes in Canada. J. Am. Heart Assoc. 2018, 7, e007535.
[54] Issa, O.M.; Roberts, R.; Mark, D.B.; Boineau, R.; Goertz, C.; Rosenberg, Y.; Lewis, E.F.; Guameri, E., Drisko, J.; Magaziner, A.; Lee, K.L.; Lamas G.A. Effect of high-dose oral multivitamins and minerals in participants not treated with statins in the randomized trial to assess chelation therapy (TACT). Am. Heart J. 2018, 195, 70-77.
Posted in Resveratrol
Add comments »